—
HPI Network > HPI - Health Policy Institute > Health System in Slovakia > 2. Organization and governance > 2.8 Regulation
2.8 Regulation
Wednesday, 04. May 2011, 22:29 — HPI
| << PREVIOUS
2.7 Health information management |
Introduction – Organization – Financing – Resources – Provision – Reforms – Assessment – Conclusions – Appendices |
NEXT >>
2.9 Patient empowerment |
- 2.8.1 Regulation and governance of third-party payers
- 2.8.2 Regulation and governance of providers
- 2.8.3 Registration and planning of health workers
- 2.8.4 Regulation and governance of pharmaceuticals
- 2.8.5 Regulation of medical devices and aids
- 2.8.6 Regulation of capital investment
In terms of regulation, the main actors in the Slovak health system are Parliament, the central government, the Ministry of Health and its subsidiary organizations as well as the self-governing regions. The Parliament as a legislative branch passes acts. The legal environment in health care is significantly influenced by general acts, including the Commercial Code, the Civil Code and the Labour Code. As the executive branch, the government and the Ministry of Health enact secondary legislation (regulations, decrees, rulings, measures, guidelines) with varying scope and different means of enforcement. The HCSA is responsible for monitoring health insurance, health care purchasing and health care providers, and also enforces the regulatory framework. The role of health insurance companies in system regulation results from their competences as purchasers of health care services. This includes maintaining the conditions of selective contracting and flexible pricing.
The Constitutional Court of the Slovak Republic rules on whether or not laws conflict with constitutionally established rights. The Constitution of the Slovak Republic stipulates that every person shall have the right to protect his or her health. Through medical insurance citizens have the right to free health care and medical equipment under the terms provided by law. The law sets the scope of free health care in general, subordinate legislation defines specific procedures. The Constitutional Court ruled that user fees for health services, which were introduced in June 2003, are in accordance with the constitutional guarantee of cost-free health care (Constitutional Court, 2005).
Another important ruling in 2008 stated that the scope of covered health care services does not have to be defined strictly by law, but can be defined also by governmental and ministerial decrees (Constitutional Court, 2008a). Later on in 2008, the Constitutional Court ruled that health insurance companies can operate as joint stock companies and said “that the legislator chose the legal form of joint stock company does not mean that the guarantee for free health care is threatened or excluded, or that such solution is in conflict with the principles of the legal state” (Constitutional Court, 2008b).
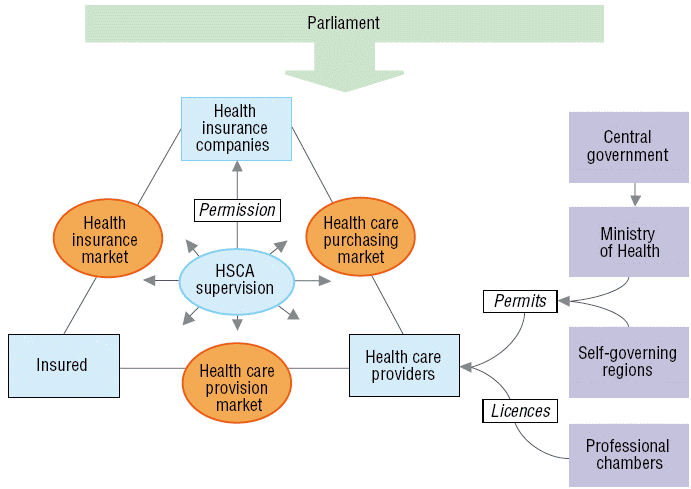
Fig. 2.2 schematically depicts the regulatory framework in Slovakia, which will be elaborated upon in the following sections.
Fig. 2.2: Regulation and supervision in the health care system
2.8.1 Regulation and governance of third-party payers
Health insurance companies providing SHI have the role of third-party payers in the Slovak health system. They operate under private law and must be established as joint stock companies. Health insurance companies are responsible for collecting contributions and purchasing health care. All health insurance companies must operate nationwide, although their market shares show significant regional variation. This results in regional differences between health insurance companies in negotiating positions vis-à-vis health care providers.
Insured Health care providers HSCA supervision Health care purchasing market Health insurance market Health care provision market Ministry of Health Self-governing regions Professional chambers Central government Parliament Health insurance companies
The HCSA issues licences for health insurance companies. Legal conditions for issuing a licence include an issued share capital of a minimum of €3.3 million and transparent staff relations. The members of the various boards (for example, of directors and trustees) are appointed by their owners. Rules apply to the shareholders, structure, staffing and purchasing policy, as well as the financial management of the health insurance company. The HCSA enforces these regulations and may impose sanctions. This may happen, for example, in cases of poor economic performance, if the health insurance company is seriously indebted or insolvent, or in cases of failure to comply with the public interest. Examples of these sanctions include imposing financial penalties, placing the company under forced management and revoking the operating licence. Health insurance companies, like all other joint stock companies, are obliged to undergo an audit of their accounting records. The health insurance company can propose an auditor but the HCSA may refuse this and assign another one. The HCSA submits biannual reports on the financial administration of health insurance companies as well as an annual budget proposal to the Ministry of Finance and the Ministry of Health. All health insurance companies must publish annual reports via the Commercial Register.
The (central) government plays an important role in regulating health insurance companies. During the preparatory process of the state budget, the government decides on additional financial sources for the system through changing the contribution rate for the state-insured. Through the Ministry of Health, it defines the (minimum) benefit package, minimum provider network, reimbursement policy, whether user fees apply and maximum waiting lists. Lastly, the Ministry of Health is the only shareholder in the largest health insurance company, the General Health Insurance Company. This enables it to influence the company’s operating policy. Moreover, due to its size the General Health Insurance Company has a strong influence over the entire health insurance market.
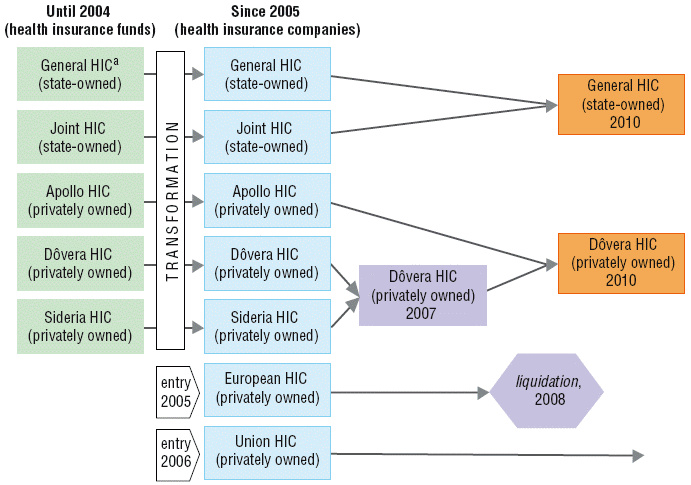
In the 2004 health reform, the public health insurance funds that had existed hitherto (operated by the state or industrial sector) were transformed into private health insurance companies, that is, joint stock companies, allowed to make profits and pay dividends to shareholders (see section 6.3.2 Transformation of health insurance funds into joint stock companies. The health insurance companies must meet all health care needs of their insured before being allowed to pay out profits to shareholders. During the three years after the reform, two profit-making health insurance companies entered the market, two companies merged to consolidate their portfolio and one ceased operations as a reaction to the changed regulatory framework from 2008. From the beginning of that year, health insurance companies were obliged to use all profits for purchasing health care in the following year. Not only was it no longer allowed for health insurance companies to pay profits to their shareholders, it was also no longer possible to recover losses from previous periods, including those associated with the acquisition campaign after entering the market. In January 2011, the Constitutional Court ruled that the profit restriction was unconstitutional and nullified it. After two more mergers, as of 2010, the market consists of one state-owned health insurance company and two privately owned health insurance companies (see Fig. 2.3). The total market share of state owned companies dropped from 76% in 2005 to 67% in 2009. Despite this, the health insurance market continues to be very concentrated.
Fig. 2.3: The health insurance market structure, 2004–2010
Timely access to health care is also regulated by the law. In general, the waiting lists should not exceed 12 months. Empirical findings indicate considerable differences in the length of waiting lists between different health insurance companies. Subordinate legislation issued by the Ministry of Health regulates only three types of waiting lists (implantations of artificial joints, implantations of artificial lenses and heart interventions). This prevents the HCSA from monitoring the overall waiting times.
2.8.2 Regulation and governance of providers
The following conditions need to be met by a health care facility in order to provide health care in Slovakia: (1) a permit to operate the facility; and (2) a licence from the relevant professional chamber for the various professionals working in the facility. Both can be requested if material, technical, staff and qualification requirements are met.
The permits for almost all in- and outpatient facilities are issued by the self-governing region. Possible disputes are settled by the Ministry of Health. The Ministry of Health issues permits for providers of emergency medical services, specialized hospitals, facilities of biomedical research, tissue units, biological banks and reference laboratories. Providers willing to perform their profession in several self-governing regions also fall under the competence of the Ministry of Health.
Permits are granted for an indefinite period of time, during which the provider is obliged to observe the legal conditions of his entry to its market. The facilities of emergency medical services are an exception; they can only obtain a permit for a period of four years based on a tender from the Ministry of Health. After winning a tender, financing from health insurance companies and an identified operating territory are secured.
Independent health care professionals who do not operate any health care facility but function as entrepreneurs may provide health care services based only on their licence to practise medicine independently.
Almost all GPs and the vast majority of specialized physicians provide health care services in their private medical practices. The state is the owner of the largest (mostly university) hospitals, almost all of which are contributory organizations. Five health care institutions were transformed into joint stock companies by the 2004 health reform. The intention to transform the rest of them was dropped after the elections in 2006. The rest are either owned by self-governing regions or private entities.
The legal form of health care providers is deregulated and shows a wide variety, including contributory organizations, non-profit making organizations and commercial companies. Irrespective of their legal form, all need to compete for contracts with health insurance companies based on quality criteria and prices. However, a total of 39 state hospitals, specialized institutions and medical institutions are in a privileged position. Since 2007, they do not have to compete with non-state providers for contracts as the government considered them pivotal in guaranteeing geographical accessibility. Health care providers are not required to be subject to external monitoring, or to publish their financial results or quality indicators.
The Ministry of Health regulates natural healing spas, natural healing resources and natural mineral waters through the State Balneal Committee. Regional public health authorities perform health impact assessments, including radiation protection in health care facilities. Pharmacies must have an expert opinion from the SIDC.
Regulating quality of provided care focuses on three components: structure, processes and results. The first component, regulation of structure, is most clearly defined. The Ministry of Health sets minimum criteria for material and technical equipment as well as qualifications and personal criteria. These criteria are a prerequisite for market entry and must be maintained continuously. Monitoring and enforcement of these criteria are the responsibility of the HCSA, professional chambers and the self-governing regions.
The second quality component, regulation of processes, is very general. The Ministry of Health requires providers to document their quality system in writing, in order to reduce shortcomings in health care provision. However, the Ministry of Health has so far not enforced this requirement. It only issues guidelines, which are neither legally binding nor enforceable. Therefore, quality systems in health care are mostly a formality or do not exist at all.
The third quality component, regulation of results, is limited to issuing quality indicators on health care providers, which serve as criteria for selective contracting. Quality indicators are published and developed by the Ministry of Health in cooperation with professional organizations and health insurance companies as well as the HCSA. The most recent quality indicators were published in February 2009. The list is rather basic and does not provide sufficient information on the quality of health care providers. According to the HCSA’s own statement, the data collected by health care providers have low validity, which results in low credibility regarding the providers’ ranking.
Suspicions of malpractice are investigated by the HCSA. If malpractice is confirmed, the HCSA can impose sanctions on the health care provider. In case of a suspected crime, the HCSA files a motion to bring the contested issue before a court for a decision. Such incidents are published by the HCSA in case report summaries.
2.8.3 Registration and planning of health workers
Each health professional is obliged to register with the relevant professional chamber and provide updates regarding his or her occupational and educational activities. Upon completion of a university education and having been issued a licence, graduated physicians are authorized to practise as physicians. Health care professionals can be providers themselves (as entrepreneurs) or employees of a provider. As providers they need both permit and licence, as employees they need only a registration from the professional chamber. A licence is also issued by the professional chamber and is a proof of qualification (education and years of practice). In order to operate an outpatient practice, a physician must then submit his or her licence to the Chief Physician of the self-governing region together with the application for a permit to operate an outpatient practice. Upon fulfilling certain requirements regarding qualification and medical equipment (technical and personnel criteria established by law) a physician is authorized to run his or her own practice. There is no system of recertification of licences in the Slovak health system. Furthermore, there is no mechanism for regulating the number of health workers in each category and specialization according to the population’s needs.
There is no central workforce planning at ministry level that takes into account future inflows and outflows of health workers in Slovakia. A Decree of the Ministry of Health has laid down the minimum personnel and technical requirements of health care facilities, but leaves it to the discretion of the health care providers to meet these requirements. In other words, human resource planning policy is largely determined through the human resource and wage policies of individual employers. The health insurance companies are formally responsible for the accessibility of health services and must ensure a minimum network of providers to facilitate access to health care services. However, in case of shortages, there are no legal mechanisms to correct the shortcomings.
2.8.4 Regulation and governance of pharmaceuticals
Before entering the market in Slovakia, pharmaceuticals must have an authorization from the European Medicines Agency (EMA), or the nationallevel SIDC. The SIDC closely monitors the safety of drugs in Slovakia and is the national competent body responsible for pharmacovigilance. The monitoring includes reporting of adverse reactions and requiring reports from pharmaceutical companies, as well pharmaceutical quality. Reports on adverse effects are submitted to the Centre of Adverse Effects Follow-up in the SIDC. The prescribing physician is obliged to report any adverse effects. The number of reports peaked in the 1980s and 1990s (with over 2000 reports annually); in the late 1990s the number of reports fell to below 500 per year, but was well above that number in the early 2000s. In 2009, there were slightly more than 1 000 reports (SIDC, 2010).
Market authorization holders are also obliged to report adverse effects of drugs. Each market authorization holder appoints a person responsible for pharmacovigilance. In addition to physicians, the reporting of adverse effects applies to pharmacists and nurses as well as patients. The SIDC has the right to suspend distribution of a pharmaceutical, withdraw a pharmaceutical from the market and, in more serious cases, suspend the registration for 90 days or terminate the registration.
General public advertising is permitted for drugs free of dazing and psychotropic effects, and over-the-counter (OTC) drugs not covered by health insurance. Advertising aimed at physicians and pharmacists has no such limitations. Vaccination campaigns, with permission of the Ministry of Health, are another exception. The contents of general public advertising may not give the impression that medical examination is not necessary or that pharmaceutical effects are guaranteed. The description of a diagnosis should not mislead or result in self-diagnostics; it should avoid florid, offensive or misleading expressions. Advertisements should not compare a drug to food, cosmetic products or consumer goods. It must be clear that the information is an advertisement, containing clear information on proper use. The SIDC is in charge of monitoring advertisements.
Based on European legislation, as well as the recommendation of the EMA in order to improve the knowledge of patients, the SIDC has created a space for patients on a website: www.sukl.sk. This space publishes a list of patient organizations. However, it has not been updated since 2007, which may reflect an approach that views including the patients’ agenda as a formality.
Reimbursement decisions
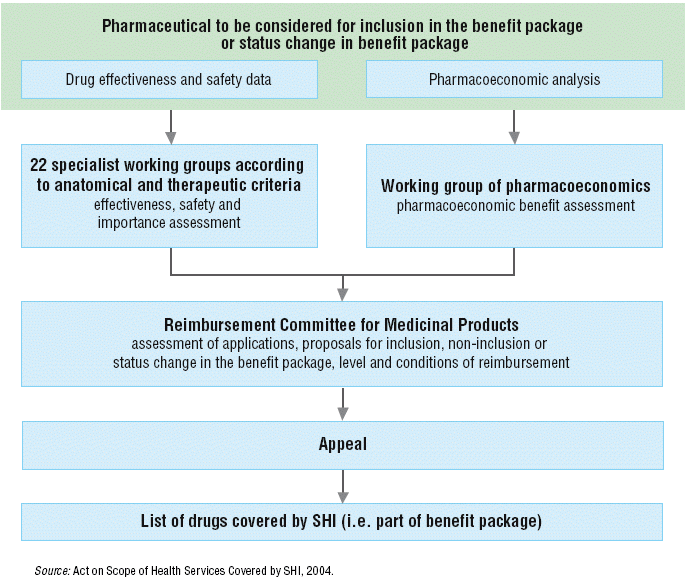
The decision as to whether a pharmaceutical will be covered by SHI, is the competence of the Ministry of Health and its Reimbursement Committee. This decision is taken after an assessment of the pharmaceutical, called the “categorization” process (see Fig. 2.4). A similar process is used for medical devices and dietary products.
First, the marketing authorization holder must submit comparative data on the pharmaceutical, including effectiveness, safety and pharmacoeconomic data. In line with recommendations from the Ministry of Health, the pharmaceutical is assessed using cost-minimization, cost–effectiveness, and cost–utility analysis. The discount rate for benefits and costs was set at 7%. The recommended threshold of a cost-effective new technology was set at €20 000 quality-adjusted life years (QALY), that is, pharmaceuticals with lower costs per QALY are considered cost-effective. In contrast, pharmaceuticals that exceed €26 500/ QALY are considered non-cost-effective. Pharmaceuticals that range between €20 000 and €26 500 per QALY will undergo further evaluation.
Second, each pharmaceutical is evaluated according to its anatomic and therapeutic classification by one of 22 specialist working groups. The working groups evaluate the effectiveness, safety and importance of pharmaceuticals. One working group evaluates the pharmacoeconomic properties of the pharmaceuticals. The results of the specialist working groups serve as background for decisions of the Reimbursement Committee for Medicinal Products. This Committee has 11 members, 3 of whom are representatives of the Ministry of Health, 5 of whom are representatives of the health insurance companies and 3 of whom are representatives of the professional public. Third, the Reimbursement Committee evaluates the therapeutic and social value of the pharmaceutical. The therapeutic value includes: (1) effectiveness; (2) safety; (3) cost–effectiveness; (4) whether it is a first or second option or adjunctive treatment; and (5) whether it is causal treatment, prophylaxis or symptomatic treatment. The criteria regarding the social value of the pharmaceutical include: (1) severity of disease; (2) impact on society if not treated (for example, spread of infection and so on); (3) social value (for example, orphan drugs); (4) risk of abuse; and (5) impact on total costs.
Lastly, the Reimbursement Committee elaborates proposals for inclusion, non-inclusion, exclusion or change of status in the benefit package, and proposals for reimbursement level, co-payment and conditions for reimbursement. The results of its decisions are published on the web page of the Ministry of Health after each meeting of the Reimbursement Committee. The applicant receives written information on the results of the reimbursement decision and the decision may be appealed.
Fig. 2.4: Pharmaceutical to be considered for inclusion in the benefit package or status change in benefit package
Pricing decisions
Slovakia operates a reference pricing system for pharmaceuticals. SHI reimbursement is set as the maximum price for a standard daily dose in the reference group of the pharmaceuticals. The definition of a given reference group is very narrow. All pharmaceuticals included in the reference group contain the same active substance and are administered uniformly. In certain cases, the Reimbursement Committee may decide to form a separate reference group for pharmaceuticals that are administered in a different form and that have a different amount of active substance per dose. The prices of pharmaceuticals covered by SHI are regulated, both in the ambulatory and inpatient sectors.
After obtaining an authorization to enter the market, the ex-factory price of the pharmaceutical is determined by the Ministry of Health through external reference pricing. The ex-factory price may not exceed the average of the six lowest prices of the same pharmaceutical sold across the EU. The prices of OTC pharmaceuticals and prescription pharmaceuticals not covered by health insurance have been deregulated.
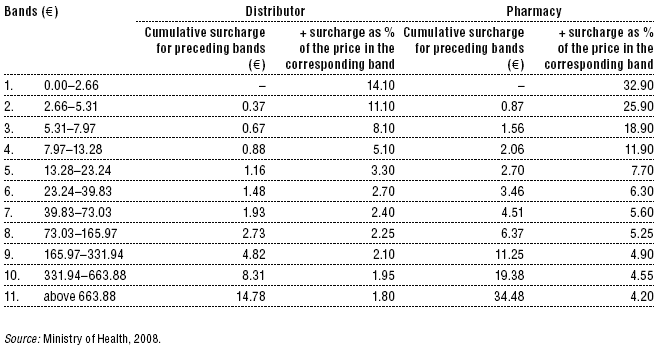
A degressive margin for pharmaceuticals and dietary foods was first introduced in Slovakia in 2004. Initially, the margins were set as a fixed percentage of the pharmaceutical price (11% for the distributor and 21% for the pharmacy). In 2004, a lower margin (10%) was established (4% for the distributor and 6% for the pharmacy) for so-called financially demanding pharmaceuticals, that is, certain high-priced pharmaceuticals that put pressure on the budget. However, what exactly constituted a financially demanding pharmaceutical was never precisely defined. The decision to include a pharmaceutical in this category was made by the Reimbursement Committee during the reimbursement decision. Since 2008, however, a more elaborate degressive system is in place, which sets margins separately for distributors and pharmacies based on the ex-factory price (Table 2.3). VAT on pharmaceuticals has changed several times since 1999. Until 1999, it was 6% after which it rose to 10% in the period 2000–2002. In 2003, VAT increased to 14% and a flat rate of 19% VAT was introduced in 2004. On 1 January 2007, the new government reduced the VAT on pharmaceuticals to 10% again. VAT on the pharmacy margin was introduced on 1 January 2004.
Table 2.3: Retail margins for pharmaceuticals
2.8.5 Regulation of medical devices and aids
Medical devices and aids are assessed through a categorization process, which is similar to the process described for pharmaceuticals. This includes the application by the marketing authorization holder of the medical device, evaluations by working groups and a reimbursement proposal prepared by the Reimbursement Committee.
2.8.6 Regulation of capital investment
Until 2004, capital investments were funded from the state budget and allocated by the Ministry of Health. Investment planning was not based on transparent relevant economic or health indicators and, as a result, the allocation of funds was a source of unpredictability. Planning and coordination of resource utilization from the EU structural funds suffer from the same problem to this day. From 2004, funds for capital investment were allocated to health insurance companies so that these could include amortization in their payments to providers. However, self-governing regions and municipalities often invest additional money in their health facilities and usually bear the investment costs in these hospitals and outpatient centres.
For the Slovak economy as a whole, the ratio of gross fixed capital formation (GFCF) as a share of GDP, which is a ratio that measures the investment rate, reaches about 25% annually. To have a stable replacement of older capital (buildings, for example), the GFCF/GDP ratio is often assumed to be at least 15%. In the 2000–2006 period, the GFCF/GDP ratio in the Slovak health care fluctuated only between 7.8 and 15.2% (Morvay, 2006). These numbers confirm that investment in Slovak health care facilities is in general very low, which leads to an outmoded infrastructure (see also section 4.1.1 Capital stock and investments).
News
The amendment of the Decree on emergency medical service
Health insurance companies returned over 400 thousand €
The HCSA received 1,647 complaints last year
A half million people will earn more
Most of public limited companies ended in the black
Debt of hospitals on premiums has grown to nearly € 105 MM
Slovak health care may miss € 250 million next year
Profits of HIC amounted to € 69 mil. last year
Owners of Dôvera paid out money but did not paid taxes
Like us on Facebook!
Our analyses
- 10 Years of Health Care Reform
- New University Hospital in Bratislava
- Understanding informal patient payments in Kosovo’s healthcare system
- Analysis of waiting times 2013
- Health Policy Basic Frameworks 2014-2016
- Analysis of informal payments in the health sector in Slovakia
- Serbia: Brief health system review
developed by enscope, s.r.o.
